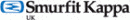CAPA Software
Intelex Corrective and Preventive Action (CAPA) software helps you to identify, anticipate and correct defects and nonconformances that prevent your products or services from meeting customer requirements.
- Maintain compliance and mitigate risk
- Store complete history of NCRs and CARs for analysis
- Reduce the Cost of Poor Quality (COPQ)
- Mobile data capture, dashboards and reports
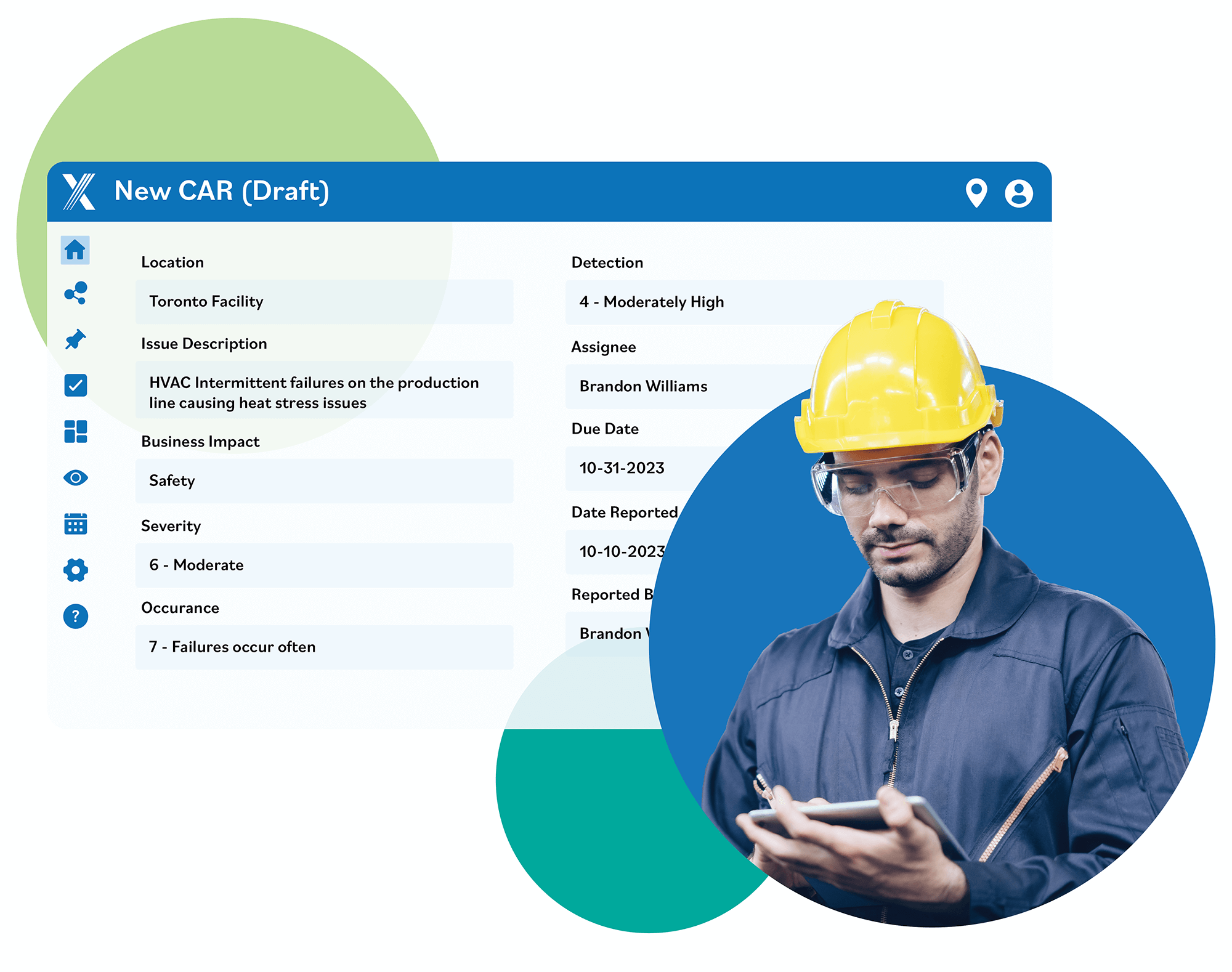
CAPA Software Creates Happy Customers
Intelex CAPA software tracks existing or potential product nonconformances to identify root
causes so you can initiate containment and develop an appropriate response
plan to prevent future defects and meet customer requirements.
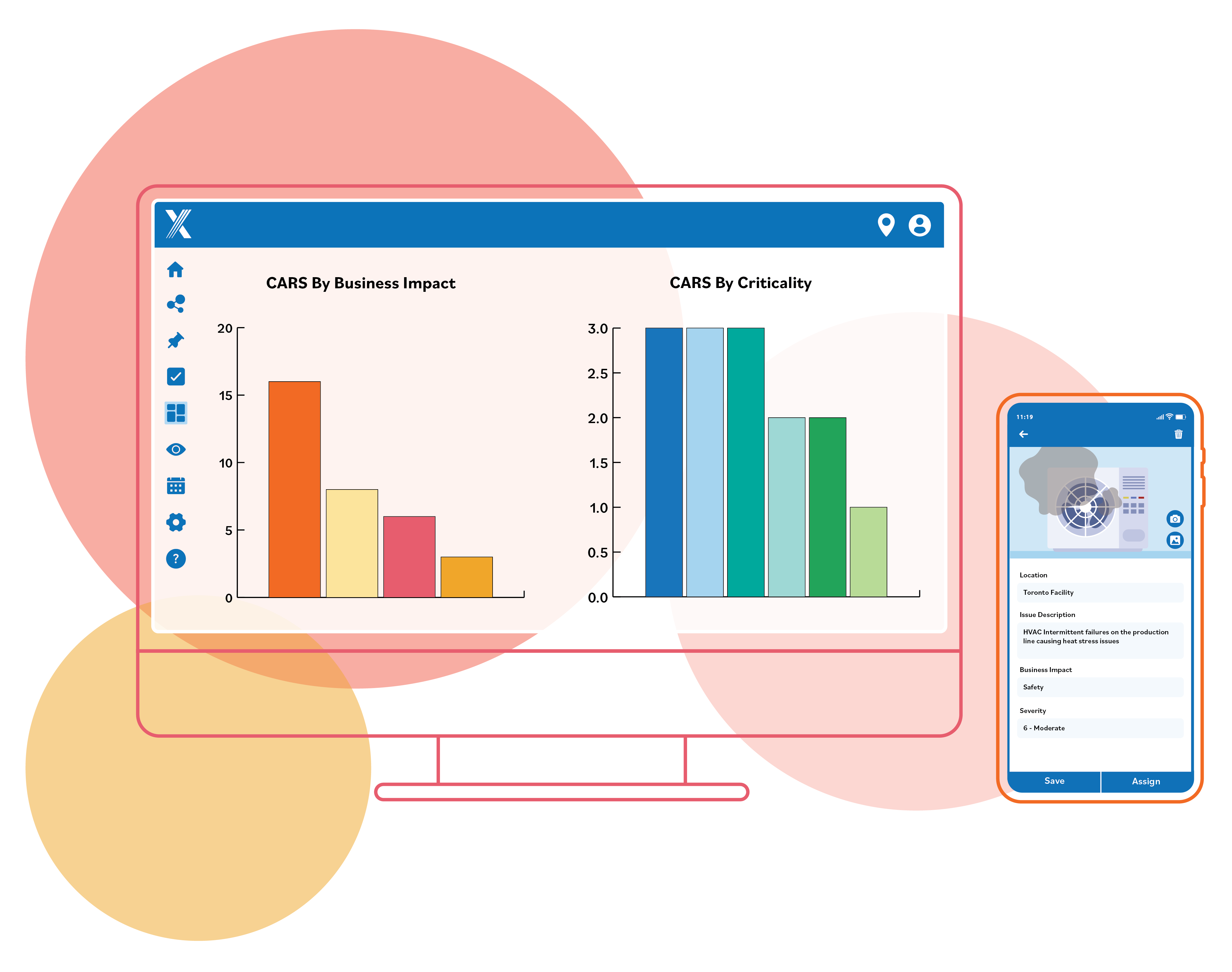
Key CAPA Software Features
Intelex CAPA software delivers a comprehensive toolset that streamlines the process of
investigating a defect, analyzing the root cause and preventing its reoccurrence.

Manage Nonconformances
Simplify the identification and documentation of new issues and defects, create new corrective action requests and prioritize requests for corrective action.
Identify Root Causes
Quickly identify stakeholders to launch Root Cause Analysis (RCA) using the built-in RCA frameworks for accelerated analysis, decision making and creation of Preventative Action Requests (PARs).

Implement Corrective Actions
Build standardized corrective action plan templates to eliminate defect reoccurrence, assess cost of quality and capture essential learnings to leverage in the future.
Continually Improve your Corporate Quality Program
Intelex CAPA software is a key piece of your corporate quality program and works with other Intelex solutions to reduce COPQ and increase productivity and efficiency.
Streamline the planning and execution of safety and quality management processes and controls.
Track quality KPIs, manage nonconformances and maintain compliance.
Keep everyone in the loop and share best practices for continuous improvement.
Expand your CAPA knowledge
with these featured resources
Trusted by over 1,400 clients and 3.5 million users worldwide

-
Based on the severity, different levels of root-causes analysis are conducted and reviewed by the management team. Corrective and preventive actions are put into the Intelex system, which are tracked until completion. This system provides us a great data set for our risk and hazards and helps us prioritize our resources.
Kanwer Khan
Vice-President, Environmental Compliance, Health, Security and Safety SUEZ North America -
Intelex’s Safety Incident Reporting Application provided such a high level of visibility into our safety performance that we were able to identify areas for improvement that would have never been uncovered otherwise. Detecting root causes and driving corrective and preventive actions, it has been a critical component to the success of our entire safety program and maintaining OHSAS 18001 conformance.
Mark Montgomery
Compliance Manager -
By using Intelex we’re able to allow people to give us problems, for us to immediately offer solutions and also then work through an investigation as to why that happens and show them that we will address the root causes of those things, we’ll come up with action plans to address any corrective actions that needs to happen.
Gary Pitts
Global Health Safety and Security Lead




CAPA Software Demo
This product demo video walks through the user-experience and core features of Intelex’s Corrective Action Reporting software application and how it allows you to identify, anticipate and correct defects and nonconformances. Drive continual improvement for your corporate quality programs and propel improved customer satisfaction and loyalty.
Watch and learn how this application can:
- Report new issues and defects
- Store complete history of NCRs and CARs for analysis
- Reduce the Cost of Poor Quality (COPQ)
- Capture data from any mobile device
- Launch CAPAs & verify completion
Send the product demo my way!
"*" indicates required fields
 1 877 932 3747
1 877 932 3747