The Intelex EHS Platform
The Intelex Platform is the backbone of our technology. It supports core EHS and Quality applications with enterprise-grade security, advanced data visualization and reporting, artificial intelligence (AI) and automated workflows. Its powerful integration capabilities allow easy connections with other business systems and applications.
Discover what's possible — schedule your platform walkthrough.
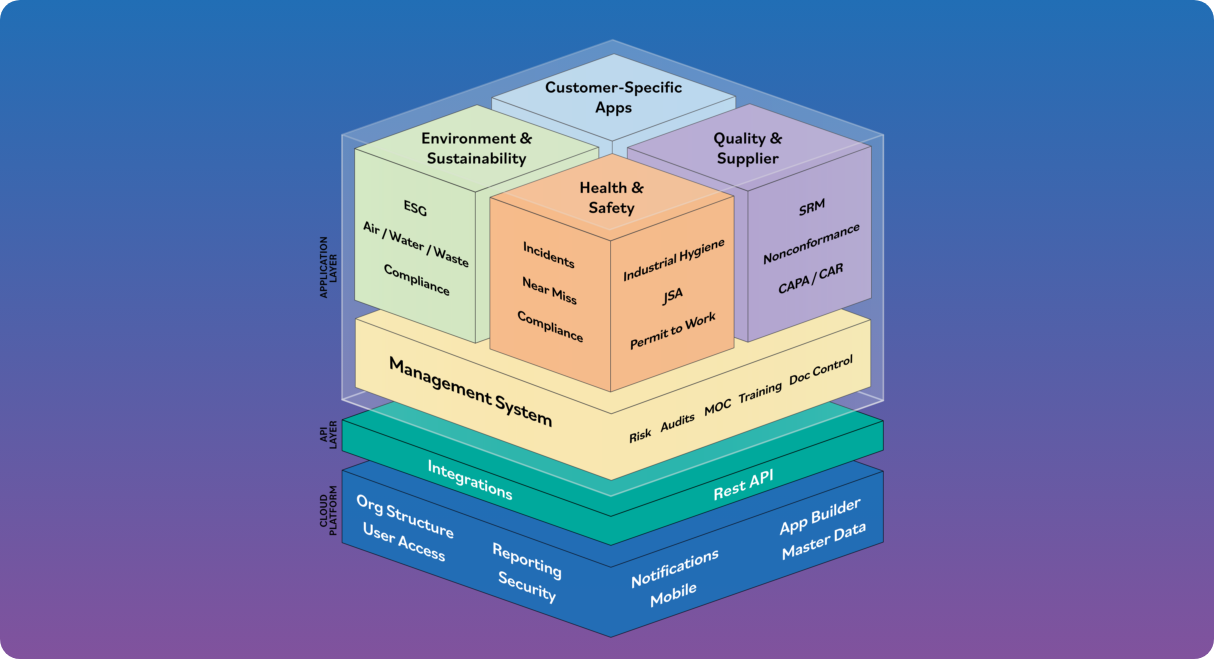
The Power of the Platform
Intelex is the most powerful software platform for EHS, Quality and ESG management. See why over 3.5 million users
worldwide choose Intelex to manage their environmental, health and safety, and quality programs.

One native platform
Our applications are built natively on one AI-enabled platform, delivering a more seamless, consistent, and reliable experience. Unlike competitors with systems patched together through acquisitions, Intelex delivers better integration and fewer complexities for smoother operations.
Configured to your needs
Intelex empowers your organization to design complex workflows, make adjustments as needed and create custom applications. As one customer put it, "You don’t have to adapt to Intelex – it adapts to you."

Exceptional EHSQ breadth
Instead of managing a mix of disparate systems, you can rely on a single system of record for Safety, Quality, Risk, Environment, and ESG.
Easy Access to Real-Time Data
Ensures your team can easily access, share and act on critical data across platforms for faster, more informed decision-making.
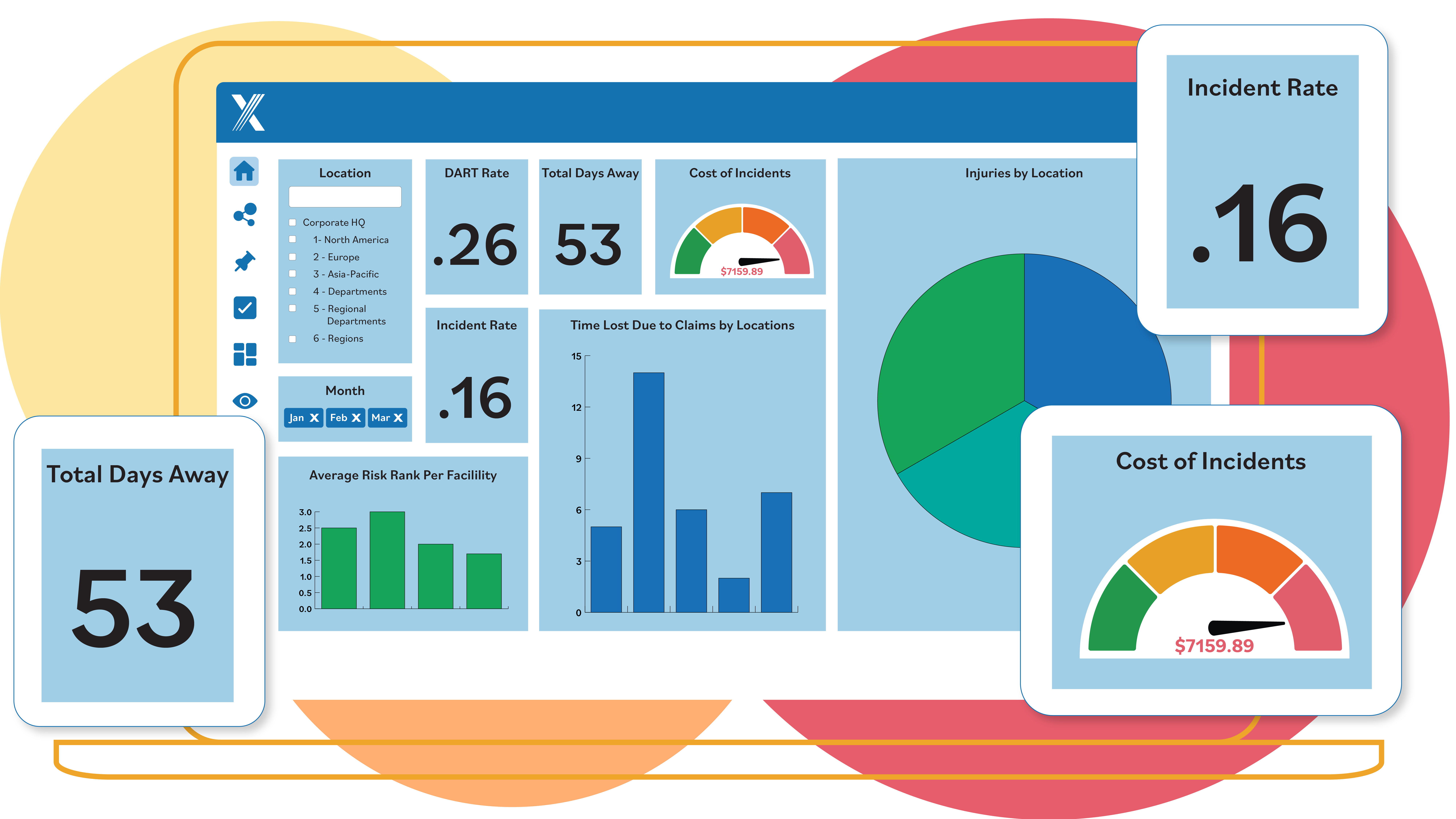
Reporting Suite
Gain real-time insights with intuitive BI tools that help you identify inefficiencies, measure initiative success and assess compliance risks. With clear visibility into your EHS and Quality programs, you can drive performance improvements and create continuous value for your organization.
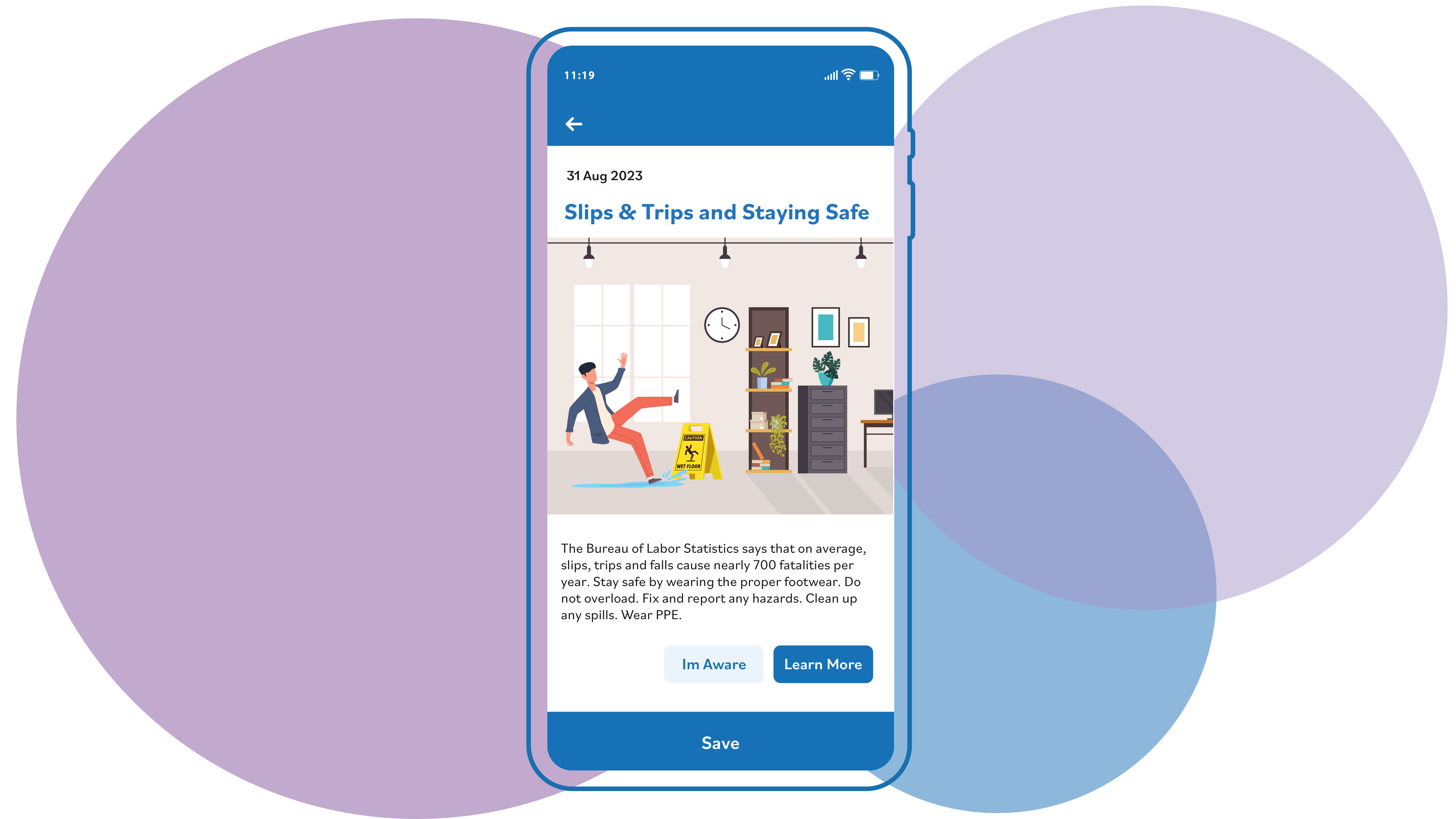
Mobile and Offline
Empower your frontline workers with intuitive tools for real-time data collection, incident reporting and compliance management. With easy access to critical EHS data, your team can stay proactive and responsive, whether online or offline, in any work environment.
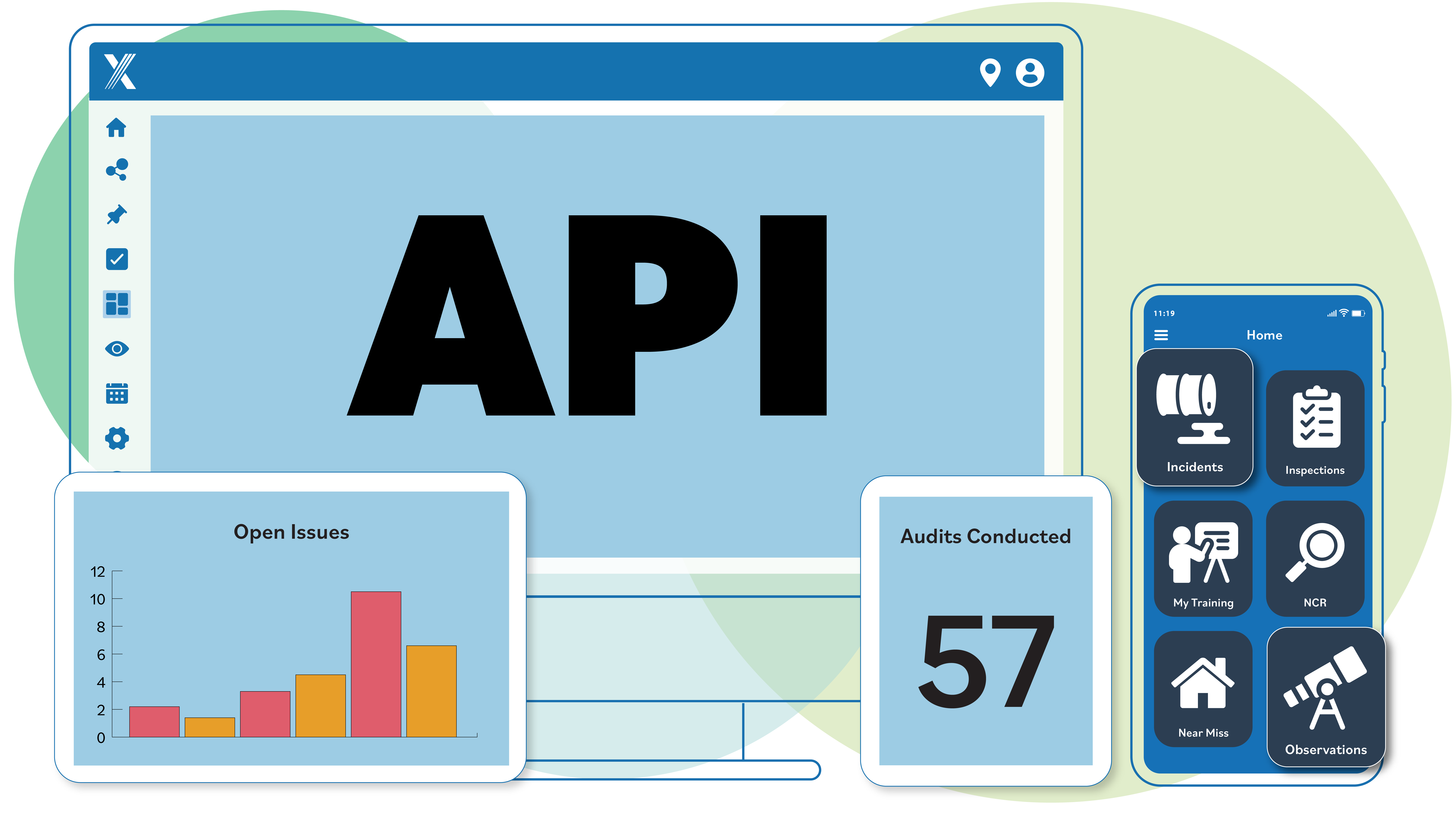
APIs, Integrations and Export
The Intelex API is built on REST, making it easy to integrate with your systems. It uses simple, predictable URLs and standard HTTP response codes, so it works smoothly with common HTTP clients. All responses, including errors, come in easy-to-use JSON format.
Enhanced Security and Customization
Intelex offers a comprehensive suite of features designed to provide enhanced security and flexibility across your entire organization.
AI Capability
Thoughtfully designed AI experiences enable users to save time and improve outcomes.

Privacy & Security
Meet stringent global standards with SOC 2, SOC 3, GDPR, HIPAA, among other certifications.

Single Sign-On (SSO)
Enable employees to use SSO credentials to authenticate and access Intelex.

Alerts & Notifications
Send media-rich bulletins to inform employees of process changes, events, and urgent alerts.

Audit Trail
Maintain a complete history of records created, modified, maintained, archived, retrieved or transmitted.
In-App Guides
Guide users through the platform with configurable on-screen tips and walkthroughs.

Multi-Language Support
Convert platform content into multiple languages.

Configurable Forms
Create, customize, and manage digital forms for data collection.

Data Service
Access and integrate data across multiple applications and systems.

E-Signature
Securely sign and approve documents with legal compliance.

Mobile & Offline Capability
Leverage iOS and Android apps to work without internet connection and sync data when reconnected.

User Access & Management
Set up users, roles, and locations to simplify program management.

Task & Action Management
Assign, track, and manage EHS-related actions and deadlines.
Application Builder
Build or extend applications with code-free development and integrated platform security.

Document Control
Store and manage document versions, approvals, and distribution in one system.

Branding & Personalization
Customize platform elements to match your company's brand.
Frequently Asked Questions
Intelex is a software platform designed to help organizations manage and improve their Environmental, Health, Safety, Quality (EHSQ) and Sustainability programs. It offers solutions for compliance tracking, incident reporting, risk management, auditing and more, helping businesses streamline operations, enhance safety and reduce risks.
The Intelex platform is a flexible, scalable technology foundation that supports a wide range of applications for EHS, Quality and Sustainability management. It provides users with tools to collect, analyze and report on key data. It enables organizations to improve their operational efficiency, ensure compliance and drive continuous improvement.
Yes, Intelex offers a powerful API that allows organizations to integrate their existing systems with the platform. This ensures seamless data sharing and enables automation of key processes across multiple business functions.
Yes, Intelex is a cloud-based platform. It provides users with secure, remote access to their data and applications from anywhere. Being cloud-based allows for real-time updates, scalability and automatic software updates without the need for on-premises infrastructure.
Interested in learning more?
Schedule a free 30 minute session with one of our experts and
explore how Intelex Software can transform your business.
 1 877 932 3747
1 877 932 3747




